Myelography PRINT
Objective
To standardize guidelines and provide information to technologists, patients, and staff on the risk and benefits of intrathecal contrast administration.
These guidelines are general recommendations but not strict policy. Treating physicians may request different preparation, procedure, and post-procedure care based on their clinical judgment as deemed appropriate. An approach that differs from the guidance in this document, standing alone, does not necessarily imply that the approach was below the standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set forth in this document when, in the reasonable judgment of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology subsequent to publication of this document. However, a practitioner who employs an approach substantially different from the guidance in this document is advised to document in the patient record information sufficient to explain the approach taken.
Appropriate Indications for Myelography1
- Demonstration of cerebrospinal fluid leak or intracranial hypotension
- Evaluation of intraspinal arachnoid webs or cysts
- Radiation therapy planning
- Non-diagnostic MR examination of the brain or spine
- Inability to perform MR safely because of surgical implant or bio-prosthesis, claustrophobia, or technical considerations such as large patient size
- Insufficient diagnostic value of MR (e.g. artifact from surgical hardware)
Relative Contraindications for Myelography1
- Coagulopathy
- Spine infection.
- Known space-occupying intracranial process with increased intracranial pressure
- Recent myelography performed within 1 week.
- Previous surgical procedure in anticipated puncture site (can choose alternative puncture site).
- Generalized septicemia.
- History of adverse reaction to iodinated contrast media and/or gadolinium-based MR contrast agents.
- History of seizures (patient may be premedicated).
- Grossly bloody spinal tap (may proceed when benefit outweighs risk).
- Hematoma or localized infection at region of puncture site.
- Pregnancy.
Withholding Medications Prior to the Procedure
Patients who are on anticoagulation therapy should discontinue these drugs for a period of time as indicated in the RIA medication guidelines for medical imaging procedures.
Screening for “seizure-threshold lowering medications” and advising patients to stop taking these medications prior to routine myelography is not indicated.
First generation (pre 1980) water-soluble nonionic contrast agents (e.g. Metrizamide) were associated with encephalopathy and seizures after intrathecal administration. Second generation (post 1980s) water-soluble nonionic contrast agents (e.g. Ioxhexol) were shown to cause significantly less severe and less frequent reactions than their first-generation counterparts. It remains unclear why second-generation agents have a lower seizure risk than first generation agents. Almost all practices now use second generation or later contrast agents.
Reasons for not stopping seizure-threshold lowering medications2-3 :
- Only a few case reports exist of seizures in patients undergoing myelography with a second generation Iohexol agent. Evidence of a relationship between these medications and newer contrast agents is tenuous at best.
- It is no longer standard of care to withhold seizure-threshold lowering medications. Fewer practices are screening for these medications compared to the past. In 2005, 63% of American Society of Neuroradiology member practitioners screened for STLMs. In 2018, only 43% of these practitioners screened for such medications.
- There is no statistically significant difference in the number of seizures related to intrathecal Iohexol contrast administration between practices that withheld seizure-threshold medications and practices that did not withhold these medications.
- More than 100 medications fall into the category of “seizure-threshold lowering medications.” Common classes include serotonin and norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, tricyclic antidepressants, and anti-psychotics. Screening for such an exhaustive list of mediations comes at a cost (disproportionate to a dubious benefit) to the hospital systems, referring clinicians, patients, and radiologists who become frustrated when appropriate procedures are cancelled or re-scheduled.
Bottom Line: While there are risks associated with any intrathecal contrast administration, these risks are very low and occur whether you withhold seizure-threshold lowering medications or not.
Suggested Contrast Agents and Usual Route of Administration
The minimum dose needed to perform the procedure should always be used. The vast majority of intrathecal administrations should be performed using a lumbar injection. However, intrathecal injections performed elsewhere in the spine may be rarely performed if there are specific anatomic or medical contraindications.
Only Isovue-M contrast is specifically indicated for myelography. Standard Isovue contrast without the -M designation is not approved for myelography. Omnipaque 140 mg I / mL and Omnipaque 350 mg I / mL are contra-indicated for intrathecal use.
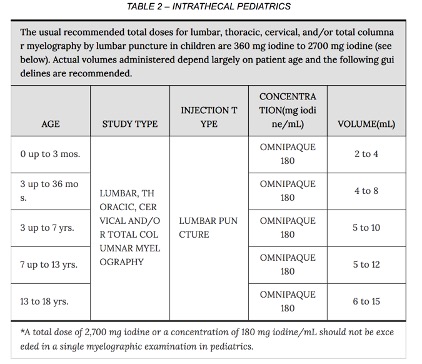
A dose of 3000 mg l in adults and 2400 mg l in children is sufficient for most myelographic procedures. (e.g. for adults less than 10 mL if using Omnipaque 300 or Isovue-M 300). The usual recommended pediatric dose range for iopamidol (Isovue-M) is 1400-2400 mg iodine. Iopamidol formulated to contain more than 200 mg l/mL should not be used intrathecally in children.
Adult
Cisternogram, cervical myelogram, thoracic myelogram, and Full Spine myelography
- Iohexol (Omnipaque) 240 mg I / mL OR iohexol (Omnipaque) 300 mg I / mL
- Iopamidol (Isovue-M) 300 mg I / mL
Adult lumbar myelography:
- Iohexol (Omnipaque) 180 mg I / mL OR iohexol (Omnipaque) 240 mg I / mL
- Iopamidol (Isovue-M) 200 mg I / mL

Isovue-M

Pediatric
All myelography procedures
- Iohexol (Omnipaque) 180 mg I / mL
- Iopamidol (Isovue-M) 200 mg I / mL

Medication Package Safety
Pharmacology, indications and use, dose and administration, and adverse reactions can be found below.
Risks of Myelography4
The following table of incidence of reactions is based on clinical studies with ISOVUE-M (lopamidol Injection) in about 686 patients.
| Isovue-M Adverse Reactions | |
| Headache | 16.4 |
| Nausea | 7.3 |
| Vomiting | 3.6 |
| Back Pain | 2.2 |
| Leg Pain | 1.4 |
| Neck Pain | 1.1 |
| Hypotension | 1.1 |
| Serious reactions (reported later in the literature but not the clinical study): seizure, meningoencephalitis, cerebral edema, etc | <<1 |
The most frequently reported adverse reactions following intrathecal administration of iopamidol are headache, nausea, vomiting, and musculoskeletal pain. These reactions usually occur 1 to 10 hours after injection, almost all occurring within 24 hours. They are usually mild to moderate in degree, lasting for a few hours and usually disappearing within 24 hours. Rarely, headaches may be severe or persist for days. Headache is often accompanied by nausea and vomiting, and tends to be more frequent and persistent in patients not optimally hydrated.
Omnipaque Adverse Reactions

Sample Consent
This is a minor procedure with benefits and risks. This procedure will benefit you by either finding an underlying cause for your current symptoms or by evaluating your current medical state. 85 to 90% people who have this procedure experience no adverse side-effects. The most common potential side-effects (such as headache) are the least serious. The most serious potential side-effects (such as infection, bleeding, seizure, nerve injury) happen very rarely. Do you consent to have this procedure?
References
- ACR–ASNR–SPR Practice Parameter for the performance of myelography and cisternography. Revised 2019.
- It Is Not Necessary to Discontinue Seizure Threshold–Lowering Medications Prior to Myelography. Krupa et al. AJNR 2019 May; 40(5): 916–919. PMID: 30948376.
- Critical Assessment of Myelography Practices: A Call for Rational Guideline Revision. Shah et al. AJNR 2018 Dec;39(12):2378-2384. PMID: 30385469.
- Online Medication Library
